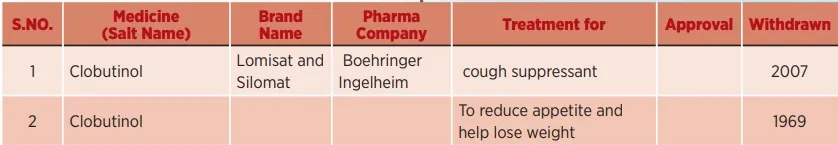
Dangerous allopathy medicines responsible for serious side effects of heart failure
(Such allopathic medicines which were banned and withdrawn from the market due to severe side effects)
On

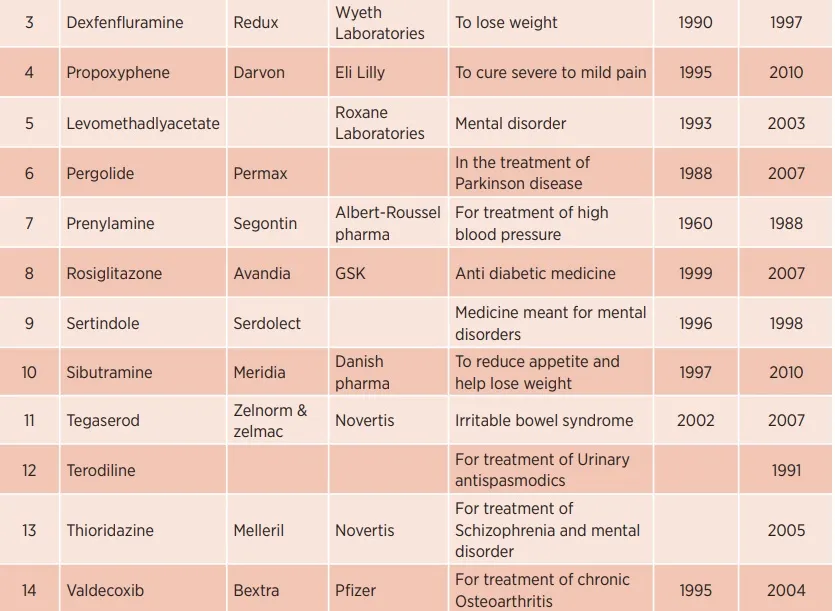
The risk of death due to stroke is also increasing among the youth. Children, old and young no one is spared from the danger of heart disease. This disease is spreading rapidly all over the world. We are presenting some living examples of this here-
First of all, let's talk about the famous television actor Siddharth Shukla, who fell victim to a heart attack at the age of just 40. Bigg Boss 13 winner and a well-known television personality Siddharth Shukla died of a heart attack on the morning of September 2, 2021. Many people could not believe at all that how Siddhartha suddenly died at the young age of 40 years.
Siddharth Shukla died of a heart attack. The night before his death, he was a little unwell, due to which he had taken some medicines. But later his health started deteriorating so he was taken to a nearby hospital. The doctor said that his condition had started deteriorating gradually and his heart beat had also become very slow. Due to which he died.
In one such incident, another well-known TV actor Siddhant Veer Suryavanshi died of a heart attack during a workout in the gym. His age was only 46 years. Siddhant fainted during the workout. Siddhant was immediately taken to Kokilaben Hospital in Mumbai, where doctors declared him dead after treatment. Siddhant proved his acting skills in many TV programs like Kasauti Zindagi Ki, Mritt Krishna-Arjun, Kusum, Grihasthi, Kya Dil Mein Hai etc. Siddhant Suryavanshi was very conscious about his fitness. For this, he used to sweat in the gym for hours every day. He also died in the gym itself. He had a heart attack during a workout in the gym.
Famous comedian Raju Srivastava also suffered a heart attack on 21 September 2022 during a workout in the gym. He was running on a treadmill at the time of heart attack. Raju fought for life and death for 42 days and breathed his last at AIIMS Hospital in Delhi.
Comedian Malkhan of the TV show 'Bhabhiji Ghar Par Hain' had collapsed while playing cricket. His nose was bleeding. He was immediately taken to the hospital where the doctor declared him dead. He had shot for his show till the night before his death. Deepesh used to be very active regarding his fitness, in such a situation, he is forced to think about death due to heart attack.
Some medicines adversely affect our heart, the use of which causes heart attack. We discussed this in the previous issue as well, let us share some more information about such medicines-
GERD or gas acidity medicine is also making heart sick
4. Salt name: Cisapride: Cisapride was discovered by Janssen Pharmaceuticals in 1980 and approved in 1993 and sold under the brand name "Propulsid", used to treat gastroesophageal reflux disease (GERD) (commonly referred to as acidity or heartburn). Also called acid reflux). It was withdrawn from the market in many countries because it caused abnormal heartbeats (arrhythmias) that could lead to death.
One case related to Propulsid is of a 2-month-old baby Sarah who used to throw up a lot of milk after drinking milk, due to which her weight was decreasing day by day. When her parents took her to the doctor, the doctor prescribed Propulsid (a medicine for acidity) and said that it would keep Sarah's spitting up under control until she was 12 months old. Sarah's problem started getting cured with that medicine and her weight also started increasing. Katie puts Sarah to sleep one evening after feeding her milk, but little does Katie know that this might be her last goodnight with her daughter. The next morning, when Katie wakes up at 6.30 am, she remembers that her daughter didn't make any noise to drink her milk tonight. She gets upset and feels that something wrong has happened, she runs to turn on the light of the room and sees her daughter. Her body turned cold and rigid, Beau comes running in and sees that his daughter is dead.
Three months after Sarah's death, Beau read an article in the newspaper. In which it was stated that Propulsid has been pulled from the market because the drug killed too many adults and children. Whatever could be found about the drug. While researching it, he soon found that many children had died of heart failure while taking the drug. The FDA never actually approved Propulsid for use in children. Doctors were using it off label in children. A survey found that 20% (about 11,600) of 58,000 children had used Propulsid. In 1993, when the FDA approved the GERD drug Propulsid, they knew that the drug could lower the QT interval (the QT interval is a measurement on an electrocardiogram that is used to assess certain electrical properties of the heart.) Can increase the problem of diabetes and can cause the death of the patient. However, FDA officials were aware of the life-threatening risk. They approved the new drug in July of the same year with a warning in the fine print on the 135th line of the drug's label. Shortly after Propulsid's release, the FDA began receiving reports of patients who experienced heart irregularities and even died of heart failure after taking the new drug. Yet the drug company denied that any of these deaths were a direct cause of the new drug. It was withdrawn from the market by the FDA on July 14, 2000.
Status in India: It was banned from the Indian market in February 2011
This problem of throwing milk has been going on in children since time immemorial. If there is no disease, just pick the child up a little and pat it on the chest, then the disease of spitting up milk can be cured up to 90% and if the child is given the ghutti of Haran and Hing according to Ayurveda, then the child's digestive system will improve. Can be cured, but the medicine we are giving to our small children even for small diseases like gas, constipation, sour belching. Those who have a very strong heart who have never smoked any kind, never drank alcohol, never had an irregular lifestyle, never had any junk food. These allopathic medicines do the work of killing even such milk-fed babies.
Arthritis medicine can also cause heart failure
5. Salt name: Rofecoxib: Rofecoxib was sold under the brand name Vioxx which was approved by the FDA in May 1999 for the treatment of osteoarthritis, rheumatoid arthritis and pain. By the time it was withdrawn from the market in 2004, it had been prescribed to 80 million people, and the Merck pharma company had sold Vioxx to the tune of $2.5 billion (Rs 18,750 crore). Merck Pharma Company also published a fake research paper The VIGOR (Vioxx gastrointestinal outcomes research) in a journal to expand Vioxx's approval data and boost sales. Before the VIGOR study was published, scientists within the Merck pharma company had grounds for suspicion that Vioxx was associated with increased cardiovascular risk.
In September 2004, Merck voluntarily withdrew Vioxx from the market due to concerns about an increased risk of heart attack and stroke associated with long-term, high-dose use. Merck withdrew the drug after revelations that it concealed negative information about Vioxx's risks from doctors and patients for more than five years, resulting in 88,000 to 140,000 cases of serious heart disease.
According to a 2004 study in the medical journal "The Lancet," 88,000 people had heart attacks after taking Vioxx, and an estimated 38,000 died.
As of March 2006, there were more than 10,000 cases filed against Merck regarding adverse cardiovascular events linked to Vioxx and the adequacy of Merck's warnings. In 2007, three years after pulling its blockbuster painkiller Vioxx off the market, Merck agreed to pay a $4.385 billion (₹36,375 crore) fine to settle nearly 27,000 lawsuits.
The above pharmaceutical company which is considered to be very responsible has printed false research papers to sell its medicine only to earn profit and you can imagine when it is about the side effects of any medicine in a country like America for 5 years to doctors, patients and FDA. You can lie, you can hide the amount of harm that the patients were suffering by lying if they can, then in a country like India, where there is no strict checking system, how much damage these foreign companies would be causing there.
Status in India: It is also banned in India since December 2004
6. Salt Name: Valdecoxib (Valdecoxibh): Valdecoxib is a nonsteroidal anti-inflammatory drug which was patented in 1995. It is sold under the brand name Bextra and was approved by the FDA on November 20, 2001 for the treatment of arthritis and menstrual cramps in women. On April 7, 2005, Pfizer withdrew Bextra from the US market on the recommendation of the FDA, citing an increased risk of heart attack and stroke and a risk of skin reaction. It was marketed by the drug company for various off-label uses.
Pfizer and its subsidiary Pharmacia in 2009 off level use of Bextra on & Upjohn 'fraud' or false branding with intent to mislead 2.3 billion dollar (Rs 18,750 crore) fine by the court imposed on.9
Status in India: The sale of Bextra was banned in India on 25 July 2005
Heart problems due to antibiotic drug
7. Salt Name: Rosiglitazone: Rosiglitazone was approved by the FDA in 1999 as an anti-diabetic drug in the US, sold under the brand name 'Avandia'. It was removed from the market in 2007 due to the risk of heart attack, it was withdrawn from the market in European markets in September 2021 and in India and Spain in 2010, while in South Africa it was removed in 2011. Rosiglitazone sales were approximately $2.5 billion (Rs 18,750 crore) in 2006. Over 13,000 lawsuits filed against GlaxoSmithKline Pharma due to adverse effects caused by rosiglitazone as of July 2010, GlaxoSmithKline Pharma (GSK) had agreed to settle more than 11,500 of these lawsuits. A 2011 study found that rosiglitazone was associated with 170 heart attacks, 649 heart failures, and 431 deaths per 100,000 people. In 2012, the US Department of Justice announced that GlaxoSmithKline agreed to plead guilty and pay a $3 billion* (Rs 22,500 crore) fine for withholding the results of two studies of the cardiovascular safety of rosiglitazone between 2001 and 2007. Was. From November 2011 to November 2013, the Federal Government (USA) did not allow 'Avandia' to be sold without a certified prescription.10
Status in India : Rosiglitazone Rezult in India (Sun pharma), Avandia (GSK) and many more used to be sold by Pharma companies but in India its sales are restricted since 2010. In India it is used to be done by 70 lakh to one crore people.10
Diclofenac increases the risk of cardic arrest
A large Danish study suggests that diclofenac is associated with serious cardiovascular complications. The Danish scientists analyzed data from the Danish National Health Service which keeps records of all Danish citizens. The study involved 1.4 million people who started taking diclofenac. They were compared to 3.9 million people taking ibuprofen, 291,000 taking naproxen, 765,000 taking acetaminophen (paracetamol) and 1.3 million taking no painkiller.
The researchers found that people taking diclofenac were 50% more likely to experience a serious adverse effect than those not taking any painkillers. Complications included heart rhythm disturbances (atrial fibrillation or flutter), clotting stroke, heart failure, heart attack, and heart damage. They were more likely to experience such side effects than people taking other painkillers.
Diclofenac is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory conditions such as arthritis. Diclofenac was introduced in 1965 by Ciba-Geigy (Novertis pharma). It came into medical use in the United States in 1988. In 2019, it was the 74th most prescribed drug in the United States, with over 10 million prescriptions.
The use of diclofenac has been banned in India, Nepal, Bangladesh and other countries since 2006 for the risk of kidney failure in animals (vultures) but is freely available in the market in the form of tablets, gel and injections for use in humans. Diclofenac is still sold worldwide under the brand names Voveran (Novartis pharma), Dictotal (Blue Cross), Gudgesic (Mankind Pharma) and many others.
Drugs withdrawn from the market for causing heart problems
Reference:
5. Source: Book: “Death by Prescription”, Author: Ray D. Strand, Published in 2003
7. https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadConsumer/banneddrugs.pdf
8. https://www.drugwatch.com/vioxx/lawsuits/
https://www.accessdata.fda.gov/drugsatfda_docs/label/2004/21647_vioxx_lbl.pdf
https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-warning-non-aspirin-nonsteroidal-anti-inflammatory
https://www.nytimes.com/2006/12/07/business/07drug.html?ex=1323147600&en=19d27b5814f1c1e8&ei=5088&partner=rssnyt&emc=rss
9. https://money.cnn.com/2009/09/02/news/companies/pfrizer_fraud_settlement.cnnw/index.htm
https://en.wikipedia.org/wiki/Valdecoxib#Side-effects_and_withdrawal_from_market
10. https://www.justice.gov/opa/pr/glaxosmithkline-plead-guilty-and-pay-3-billion-resolve-fraud-allegations-and-failure-report
https://www.thehindu.com/news/national//article59927065.ece#:~:text=The%20Government%20has%20banned%20the,the%20drug%20causing%20cardiac%20problems.
11. Source: doi:10.1136/bmj.j1358 (also Published in British Medical Journal)
12. https://en.wikipedia.org/wiki/List_of_withdrawn_drugs
S. No.1 - https://en.wikipedia.org/wiki/Clobutinol, S. No.- 5,6,10,11 - DOI:10.1002/pds.2155,
S. No.- 13- DOI:10.1002/pds.3346, S. No.- 2,3,7,9,12 DOI:10.1177/009286150103500134
Tags: patanjali patanjali wellness yog sandesh pharma mafia medical mafia education india bharat university of patanjali bharat swabhiman patanjali yog samiti swami ramdev ji maharaj patanjali gurukulam acharyakulam patanjali research institute Acharya balkrishna patanjali yogpeeth patanjali yog sandfdesh yog gram yog guru haridwar devbhumi mahila patanjali yog samiti patanjali ayurvedic hospital pharama conspiracy
लेखक
Related Posts
Latest News
01 Oct 2024 17:59:47
ओ३म 1. सनातन की शक्ति - वेद धर्म, ऋषिधर्म, योग धर्म या यूं कहें कि सनातन धर्म के शाश्वत, वैज्ञानिक,...



















.jpg)
.jpg)
